In this report of results from a survey of international uveitis experts, the authors provide guidance on the use of immunomodulatory therapy for systemic treatment of non-infectious uveitis during the coronavirus disease-2019 (COVID-19) pandemic.
Noninfectious uveitis is usually treated with corticosteroids and conventional immunosuppressive agents. Biologics are used when long-term treatment is required and a corticosteroid-sparing approach is necessary. One of the most important concerns related to immunomodulatory therapy is the increased risk of infections, as these drugs act by limiting the patient's immune responses. Patients who may be at additional risk of infection with coronavirus and/or a more severe course of, or even fatality from, COVID-19. Therefore, during the pandemic, there is an urgent need for guidance on how to manage patients with uveitis.
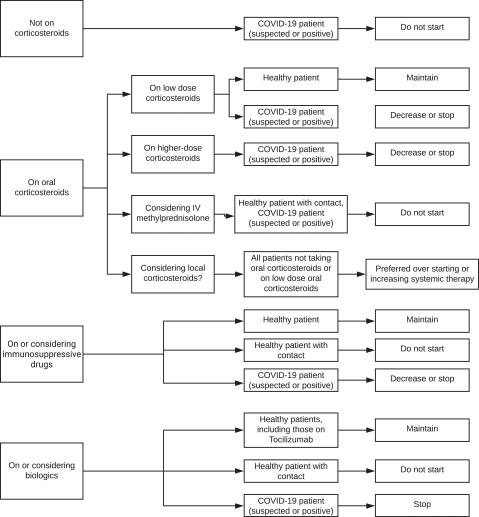
The following guidance was based on an analysis of the survey responses from 139 uveitis experts. Consensus recommendations were determined when 75% of respondents agreed with a survey statement. In the setting of COVID-19 pandemic, there was an overall consensus the use of systemic corticosteroids should be avoided in sick patients, and that local therapy (regional corticosteroid injections) should be preferred to systemic treatment in all patients, irrespective of their risk for coronavirus infection and health, except in healthy patients who are not already on corticosteroids. Experts also agreed that conventional immunosuppressives and biologics should not be started, and should be decreased or stopped in patients already on them. Interestingly, the authors noted that studies of past SARS-CoV and Middle East respiratory syndrome coronavirus outbreaks, and recent studies of COVID-19, have not associated immunosuppressed status with increased risk of adverse outcomes.
The authors make special mention tocilizumab, a biologic used for the treatment of uveitis. Interleukin-6 seems to have a crucial role in the pro-inflammatory storm that can happen in some patients with COVID-19, and interference with the interleukin-6 pathway might be a potential therapeutic strategy. Tocilizumab is a recombinant humanised anti-human IL-6 receptor monoclonal antibody that inhibits interleukin-6 signal transduction and has been proven effective in limited clinical trials in patients with severe COVID-19 disease. Given the limited information about the use of tocilizumab for noninfectious uveitis in a patient with COVID-19, however, the consensus recommendation was to only continue tocilizumab in otherwise healthy patients who are at low risk for coronavirus infection.
The authors note that these recommendations were drafted urgently, in response to the pandemic, and that the recommendations could change as research leads to a better understanding of COVID-19 and how it impacts patients on immunomodulatory therapy for uveitis and other conditions.
Agrawal R, Testi I, Lee CS et al. Evolving consensus for immunomodulatory therapy (IMT) in non-infectious uveitis (NIU) during the COVID-19 pandemic. British J Ophth. 2020.