In this article, a group of authors representing 16 different institutions (including our own Dr. Cecilia Lee) have laid out a framework for standardization of retinal imaging data collection for Alzheimer's disease biomarker research studies. Many labs are doing research in this area, but they often use different imaging modalities and protocols, making it challenging to compare data between studies. Because these studies often have small numbers of subjects by necessity, standardizing data collection will also enable researchers to collect data into a larger database, allowing them to observe larger trends in the data.
The retina is an extensive of the nervous system in the brain, and examination of the retina using optical coherence tomography (OCT) provides extensive structural information about the health of the retinal neurons and supporting tissue. Changes in the retina related to aging or disease may be connected to similar changes occurring in related neurological structures in the brain. In this way, the eye provides a sort of "window" into the brain, via noninvasive imaging techniques that can be performed in a regular ophthalmology clinic.
In this article, the authors propose a framework for a “minimum data set” for use in retinal imaging across laboratories, which was based on the design of a current multi‐site longitudinal observational trial, the Atlas of Retinal Imaging Study (ARIAS). The goal of this framework is to share reproducible methods that can be used across laboratories to aggregate data, cross‐validate findings, and accelerate the development of sensitive and specific retinal biomarkers for the early detection of Alzheimer's pathologic change.
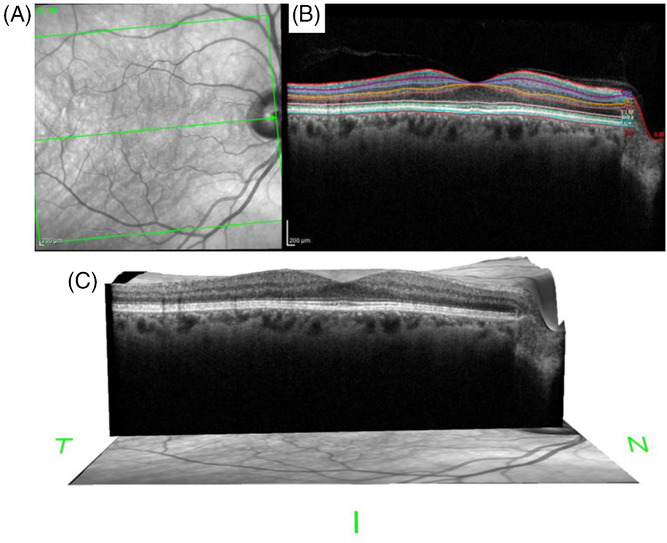
The proposed framework includes specifications about how to obtain retinal imaging data when assessing patients in studies pertaining to cognitive aging and dementia The guidelines include information about the location of the retinal structural measurements obtained using OCT, considerations about instrument variability between research centers, imaging protocols (eye dilation, etc), and specifications for other imaging modalities including autofluorescence imaging (to detect amyloid beta) and OCT angiography (to asses the retinal microvasculature). Each section includes a discussion of the appropriate outcome variables, based on the current understanding of the retinal pathology associated with Alzheimer's disease and other types of dementia.
If implemented, the data obtained from future studies will be collecting in a standardized format, allowing researchers to collaborate more effectively towards the goal of identifying and validating retinal biomarkers of Alzheimer's disease.
Alber J, Arthur E, Sinoff S, DeBuc DC, Chew E, Douquette L, Hatch W, Hudson C, Kashani A, Lee CS, Montaquila S, Modzbar S, Cuhha LP, Tayyari F, Van Stavern G, Synder PJ. Minimum data set for SD‐OCT retinal imaging and analysis from the Atlas of Retinal Imaging in Alzheimer's Study. Alzheimers Dement. Volume 12, November 2020, Page e12119. doi.org/10.1002/dad2.12119

